news release - April 11, 2025
Flaviviruses in the World and Europe
The FLAVIVACCINE project focuses on infections caused by viruses from the Flaviviridae family, particularly those transmitted by mosquitoes. Its goal is to address the growing global threat posed by flaviviruses, including dengue, yellow fever, Zika, and West Nile virus. Importantly, the FLAVIVACCINE candidate also holds potential to protect against other flaviviruses such as Japanese encephalitis.
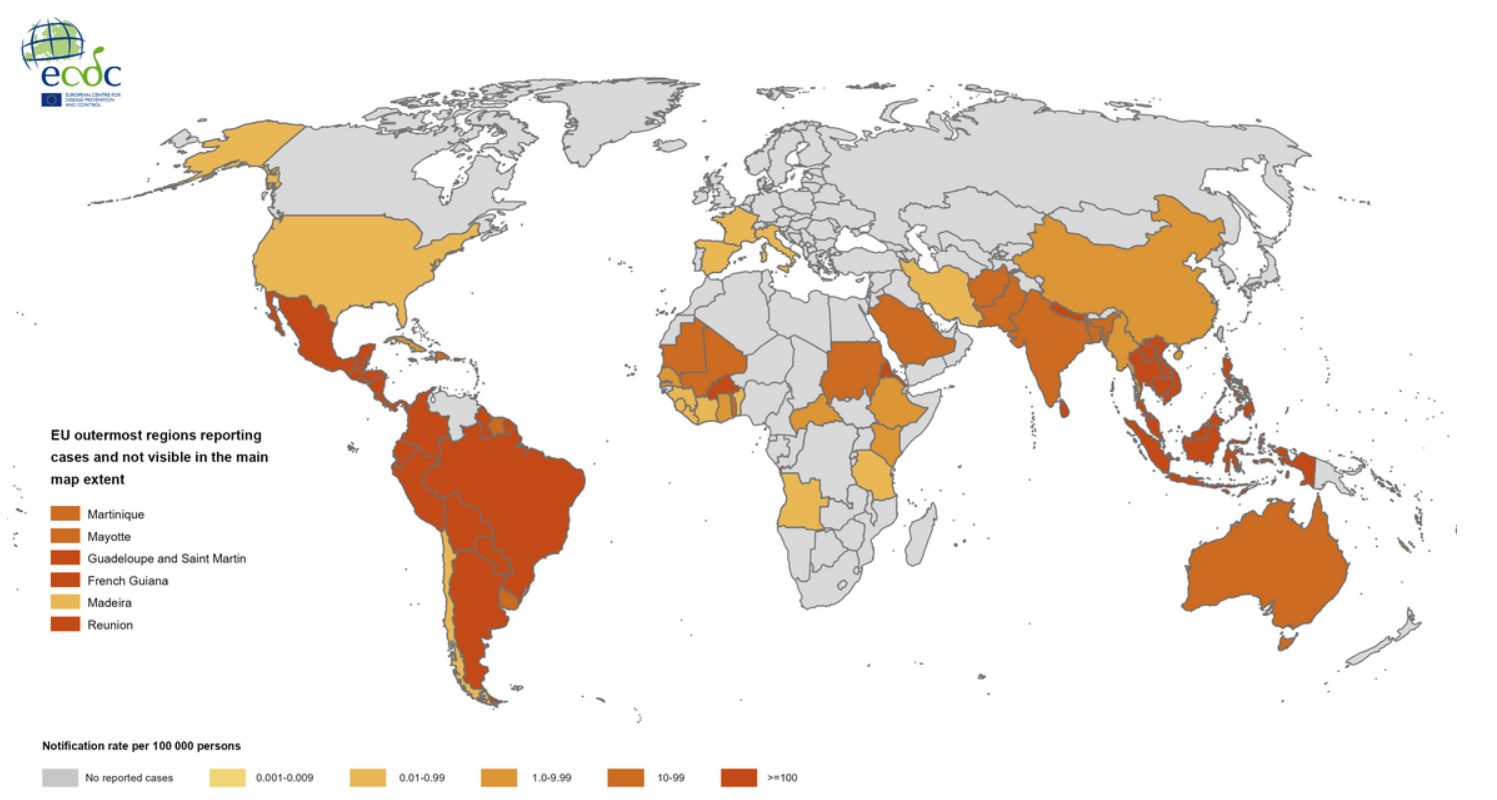
According to the European Centre for Disease Prevention and Control (ECDC), the number of dengue cases worldwide has increased significantly. In 2023, there were 6 million reported cases globally. By 2024, that number had more than doubled to 14 million, resulting in approximately 10,000 deaths.
In Europe, dengue cases are also on the rise. In 2024, a total of 304 cases were reported, compared to 128 in the previous year. The majority occurred in Italy, with 213 cases, followed by France with 83, and Spain with 8. While most of these cases were imported, there is a growing trend of autochthonous, or locally acquired, infections. Warmer environmental conditions are a major contributing factor. In particular, the ambient temperature is one of the key environmental elements influencing mosquito-borne transmission of dengue. The optimal daily average temperature for dengue virus transmission by Aedes albopictus in temperate regions of the northern hemisphere is between 24 and 26 °C. All recorded outbreaks of locally acquired dengue in mainland Europe have occurred between June and November.
Beyond dengue, FLAVIVACCINE also targets other important flaviviruses. In the Americas—including North, Central, South America, and the Caribbean—there were 50 reported cases of yellow fever in 2024, resulting in 24 deaths. West Nile virus was detected in 19 European countries, including Italy, Greece, and Spain, with a total of 1,436 cases and 125 deaths. Zika virus infection was less common, with 80 cases reported in Europe in 2023, the highest number of which were found in Spain.
According to a Forbes article by Dr. Stephen J. Thomas, despite the rising burden of dengue, several vaccine developers are scaling back their engagement in disease control. Currently, there are three main dengue vaccine candidates. Dengvaxia was one of only two licensed dengue vaccines and the only one approved in the United States. However, the company recently announced it would cease production, with remaining stock expected to run out by August 2026.
Another licensed vaccine is TAK-003, also known as Qdenga, developed by Takeda. This vaccine has a better safety profile than Dengvaxia, but its effectiveness appears limited to dengue-1 and dengue-2. It shows little to no protection against dengue-3, and its efficacy against dengue-4 remains unclear. Qdenga is licensed in the European Union but the application was withdrawn in the United States (Thomas, 2024, December 18).
A third candidate, Butantan-DV, was developed by Brazil’s Instituto Butantan using technology licensed from the U.S. National Institutes of Health. A large-scale trial involving around 16,000 participants has shown that the vaccine is well tolerated and provides protection against dengue-1 and dengue-2. The company has submitted the final required documentation to Brazilian regulators for vaccine approval.
Although there are vaccines available for individual flaviviruses like dengue and yellow fever, there is currently no single solution that offers broad protection. This is where the FLAVIVACCINE project stands out. By aiming to protect against multiple flaviviruses within one formulation, it represents a promising and unique approach in the fight against mosquito-borne diseases.